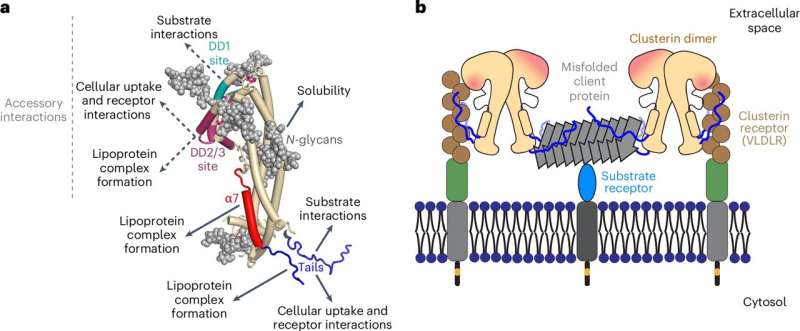
Late-onset Alzheimer’s disease (LOAD) is the most common form of dementia, with symptoms appearing after age 65. Since carriers of clusterin risk alleles have an increased likelihood of developing LOAD, the associated clusterin protein is of interest to researchers. In order to better understand the function of the associated protein, researchers at the Max Planck Institute of Biochemistry have deciphered the molecular basis for the chaperone function of clusterin.
The researchers were able to determine the crystallographic three-dimensional structure of human clusterin for the first time and discovered that two disordered, hydrophobic peptide tails are crucial for the diverse binding and protective functions of clusterin. The findings have now been published in the journal Nature Structural & Molecular Biology.
Structure of clusterin: A key protein against neurodegenerative diseases
A team led by Patricia Yuste-Checa, Andreas Bracher and F.-Ulrich Hartl, Director and Head of the Department of Cellular Biochemistry, has now used X-ray crystallography to elucidate the three-dimensional crystal structure of human clusterin for the first time. Knowing how the atoms are arranged in the protein allows conclusions to be drawn about its general mode of action and chaperone function.
The study shows that clusterin is composed of three different domains. Of particular interest are two disordered, hydrophobic peptide tails that give the protein its remarkable versatility. Yuste-Checa, first author of the study, explains, “The structure of the peptide tails is comparable to that of small heat shock proteins. These are molecular chaperones that prevent protein clumping inside cells, while clusterin functions outside of cells.”
Proteins fulfill a wide variety of functions in cells and must be precisely folded to do so. Incorrect folding can lead to the formation of harmful aggregates—a typical characteristic of many neurodegenerative diseases such as Alzheimer’s or Parkinson’s.
Molecular chaperones such as clusterin play a central role in preventing such misfolding. Clusterin, also known as apolipoprotein J, has been known since the 1980s as an abundantly secreted glycoprotein. However, until now, there has been no detailed understanding of the molecular functioning of this versatile protective protein.
Protection against protein aggregation
“Clusterin acts in the extracellular space: it binds to misfolded proteins, including the aggregation products of amyloid beta, tau, and α-synuclein, which are typical of diseases such as Alzheimer’s or Parkinson’s and prevents them from aggregating further,” Yuste-Checa states.
“In the study, we were able to show that the hydrophobic, i.e., water-repellent, peptide tails of clusterin are essential for the protective function. After we had biotechnologically modified or removed the hydrophobic amino acids in the peptide tails, we lost the chaperone activity, i.e., the protective function against amyloid beta aggregation.” Binding to cell surface receptors and the formation of lipoprotein complexes also appears to be mediated by the peptide tails.
Significance for medicine
The new insights into the structure and function of clusterin are medically relevant. Andreas Bracher says, “Numerous functions have been demonstrated for clusterin, initially as a cell aggregation factor, later as an apolipoprotein, inhibitor of the complement system, molecular chaperone, and anti-apoptotic factor.
“It is known that clusterin binds extracellular amyloid beta plaques and that clusterin levels in cerebrospinal fluid are elevated in Alzheimer’s disease. Deciphering the structure and mechanism of clusterin gives us new insights into the extracellular control mechanisms of protein stability and will hopefully be helpful for clinical research and future treatment of neurodegenerative diseases.”
More information:
Patricia Yuste-Checa et al, Structural analyses define the molecular basis of clusterin chaperone function, Nature Structural & Molecular Biology (2025). DOI: 10.1038/s41594-025-01631-4
Provided by
Max Planck Institute for Biochemistry
Citation:
3D structure of human clusterin sheds light on Alzheimer’s risk factor (2025, September 5)
retrieved 5 September 2025
from https://medicalxpress.com/news/2025-09-3d-human-clusterin-alzheimer-factor.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.